Sodium metasilicate pentahydrate Production process
The synthesis methods of sodium metasilicate include spray drying method, melt solidification crystallization method, one-time granulation method and solution crystallization method
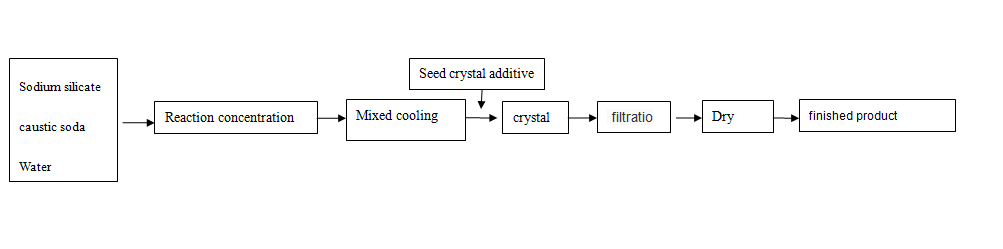
The crystallization process has the characteristics of less equipment investment, low production cost and stable quality. The process flow is shown as follows
2.1 Effect of crystal concentration
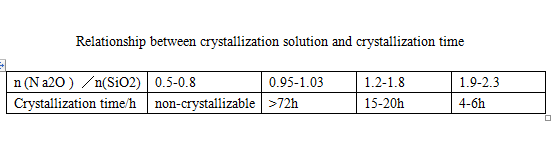
Sodium metasilicate pentahydrate is prepared by solution crystallization process. According to the phase diagram [3], the concentration of its crystallization solution (Na2O+SiO2) should be controlled as long as
Sodium metasilicate pentahydrate can be produced in the range of 25%~28% (mass fraction). However, there are enough N a2O and SiO 2 in the solution
The number is mutually affected. The mass fraction of 8i02 is high, the crystallization period is long, and the chain ratio of n (Na2O)/n (SiO2) directly used is 1,
The solution containing 58% mass fraction is crystallized, and the crystal seed is added. The crystallization cycle takes 72~120h; High content of Na2O
The speed is faster, but the faster crystallization speed is easy to cause fine crystal particles, more Na2O entrained by crystal growth, and the product modulus is difficult to reach
To requirements, see Table 1.
2.2 Seed effect
In the crystallization process of sodium metasilicate, in order to control the crystal quality and obtain products with uniform particle size
Add crystal seeds with appropriate particle size and quantity, and gently stir the whole process to make the crystal seeds more evenly suspended in the whole solution
Reduce the amount of secondary nucleation, so that the crystallized material only grows on the surface of the crystal seed
The amount of seed crystal added depends on the quality, variety and particle size of the product that can be crystallized during the whole crystallization process and the desired product
The granularity of. Assuming no primary nucleating seed is generated in the process, the number of particles in the finished product is equal to the number of newly added artificial seed particles
Mp/KvpLp3=Ms/KvLs3P, then M s=Mp (Ls/Lp) 3
Where: M s, M p —— quality of crystal seed and finished product; Ls, Lp —- average particle size of crystal seed and finished product; K v, P metasilicic acid
Physical property constant of sodium.
For the crystallization process of sodium metasilicate aqueous solution, according to the analysis of crystal phase transition, due to the narrow width of its metastable zone, it is easy to enter
In the unstable area, seeds with a particle size of 0.1-0.2mm are generally added. If the average particle size of the finished product is required to be 1mm Considering the unavoidable
The nucleation quantity of the free solution itself is 40%~60% of the mass fraction when 0.1 m crystal seeds are actually added
2.3 Temperature control influence
The crystallization process of sodium metasilicate pentahydrate is sensitive to temperature, and its crystal growth needs to go through an induction process, which is adopted between 50-60 ℃
The total amount of crystal nuclei is controlled by adding crystal seeds to the solution, and then the crystal grows at a uniform rate under a relatively constant temperature and supersaturation. In the later stage of crystallization, cool down at the rate of 1 ℃ per minute to make the crystal grow rapidly, and separate the material when it reaches 38-48 ℃
2.4 Impact of other additives
In order to facilitate the separation of free water and crystal during the separation operation, the ratio of 0.005%~0.015% of the total amount shall be taken 0.5h before the end of cooling
The surface tension between crystal and water can be reduced by adding dodecyl sulfonic acid surfactant once, which can free the wet sample
Water drops below 4% for drying and storage
Post time: Oct-13-2022